ICSE Revision Notes for The Periodic Table Class 9 Chemistry
Chapter Name | The Periodic Table |
Topics Covered |
|
Related Study |
Approaches to Periodic Classification of Elements
Dobereiner’s Triads
In 1829, Dobereiner classified elements with similar chemical properties into groups of three called Triads. He noted that the atomic weight of the middle element in a triad is the arithmetic mean of the other two. This is called Dobereiner’s Law of Triads.
|
7Li11Na39K |
At Wt. of Sodium = (7 + 39)/2 = 23 |
Reasons for discarding the Law of Triads
- Dobereiner, however, could not arrange all the known elements at that time in the form of triads.
- The law was not applicable even in the same family.
Newlands’ Law of Octaves
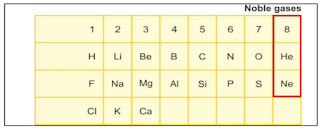
Newlands proposed a Law of Octaves which states, ‘When elements are arranged in the increasing order of their atomic masses, the properties of the eighth element are similar to the first’. Therefore, his classification was known as Newlands’ Octaves.
|
Western Music Do |
Re |
Me |
Fa |
So |
La |
Ti |
|
Indian Music Sa |
Re |
Ga |
Ma |
Pa |
Dha |
Nee |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
H |
Li |
Be |
B |
C |
N |
O |
|
F |
Na |
Mg |
Al |
Si |
P |
S |
|
Cl |
K |
Ca |
|
|
|
|
Merits of Newlands’ Classification
- This system worked well with the lighter elements. For example, Lithium, Sodium and Potassium were brought together.
- It relates the properties of the elements to their atomic masses.
- For the first time, it was shown that there is a distinct periodicity in the properties of elements.
Reasons for discarding Newlands’ Theory
1. The idea of Octaves could not be applied to noble gases. After their discovery, noble gases became the ninth element, and not the eight, which had similar properties. For example, Helium or Neon became the ninth element, and not the eighth element, with similar properties.
2. This classification could not be applied to heavier elements, i.e. those lying beyond Calcium. Every eighth element after Calcium did not show properties similar to the first one. 3. As more elements were discovered, they could not be fitted into Newlands’ Octaves.
Mendeleev’s Periodic Law
The physical and chemical properties of elements are a periodic function of their atomic masses.
Essential features of Mendeleev’s Periodic Table
- There are eight vertical columns called groups. They are numbered from I to VIII. Groups I to VII are further divided into two subgroups A and B.
- All the elements of a sub-group have similar properties and show same valency, which is equal to the group number.
- There are seven horizontal rows, known as periods. They are numbered from 1 to 7. As one moves from left to right in a period, there is a gradual change from metallic to non-metallic properties.
Merits of Mendeleev’s Table
- Grouping of elements: Mendeleev generalised the study of the elements then known as the study of a mere eight groups.
- Gaps for undiscovered elements: To make sure that elements with similar properties lie in the same group, Mendeleev left some gaps in the periodic table. These gaps were left for subsequent inclusion of the elements not known at that time. The elements were discovered later.
- Prediction of properties of undiscovered elements: Mendeleev could predict the properties of unknown elements on the basis of the properties of the elements lying adjacent to the vacant spaces. They were named as Eka-boron, Eka-aluminium and Eka-silicon. The properties of these three elements were similar to the actual elements discovered later. Eka-boron was similar to Scandium, Eka-aluminium was similar to Gallium and Eka-silicon was similar to Germanium.
- Incorrect atomic mass was corrected: He was able to correct the values of the atomic masses of elements such as Gold and Platinum, when he placed these elements according to the similarity in their properties.
Defects in Mendeleev Periodic Table
1. Anomalous pairs
The following pairs of elements did not follow Mendeleev’s Principles:
Cobalt (58.9) with higher atomic mass precedes Nickel (58.6) in the periodic table. Tellurium (127.6) with higher atomic mass than Iodine (126.9) is also placed before it in the periodic table.
2. Position of Isotopes
The isotopes of an element are atoms of that element with similar chemical properties but different atomic masses. According to Mendeleev’s Periodic Law, isotopes of an element must be given separate places in the periodic table because they have different atomic masses.
3. Grouping of chemically dissimilar elements
Elements such as Copper and Silver bear no resemblance with the alkali metals such as Lithium and Sodium, but they have been placed together in the first group.
4. Separation of chemically similar elements
Elements which are chemically similar such as Gold and Platinum have been placed in separate groups.
5. Electronic arrangement
It does not explain the electronic arrangement of elements.
6. The position of hydrogen
Hydrogen was not given a fixed position. It was considered in Group IA as well as in Group VIIA, because it forms a positive ion as in HCl as well as a negative ion as in NaH.
Modern Periodic Law
The physical and chemical properties of elements are the periodic functions of their atomic numbers.
Main features of the Modern Periodic Table
- The modern periodic table contains 7 horizontal rows called periods and 18 vertical columns called groups.
- Apart from the seven rows, there are two additional rows placed separately at the bottom of the table.
- The entire table is divided into four blocks—s-block, p-block, d-block and f-block.
- The modern periodic table is also called the Long Form of the Periodic Table.
- 5The similar properties which recur after a regular interval are called periodic properties.
Anomalies in Mendeleev’s Classification of Elements
Position of Isotopes
All the isotopes of an element have the same number of protons, so their atomic number is also the same. Because they have the same atomic number, they can be put in one place in the same group.
Position of Argon and Potassium
Argon with an atomic number lower than Potassium was placed after Potassium in the periodic table. According to the Modern Periodic Law, the elements are arranged in the order of their increasing atomic number. Thus, Argon with a lower atomic number should come first, followed by Potassium with a higher atomic number than Argon.
Periodicity in the Modern Periodic Table
In the modern periodic table, properties reappear at regular intervals due to similar electronic configurations. This happens because the elements in a group have the same number of valence electrons, and the elements in a period show a gradual increase in the number of valence electrons.
Salient features of the Modern Periodic Table
Groups
- The modern periodic table has eighteen vertical columns known as groups, arranged from left to right in the order: IA, IIA, IIIB, IVB, VB, VIB, VIIB, VIII (three columns), IB, IIB, IIIA, IVA, VA, VIA, VIIA and Zero.
- A group is determined by the number of electrons present in the outermost shell.
- Groups 1, 2 and 13 to 17 [IA to VII A] are called normal elements.
- Groups 3 to 12 [IB to VII B and VIII] are called transition elements.
- Group 18 [Zero], at the extreme right, contains noble or inert gases.
- Reactive metals are placed in groups 1[IA] and 2[II A].
- Transition elements [metals] are placed in the middle.
- Non-metals are placed in the upper right corner of the periodic table.
Periods
- The horizontal rows of elements in a periodic table are called periods. There are seven periods in the long form of the periodic table.
- The elements in a period have consecutive atomic numbers.
- The first period contains 2 elements and is called a very short period.
- The second period contains 8 elements and is called a short period.
- The third period contains 8 elements and is also a short period.
- The fourth period contains 18 elements and is called a long period.
- The fifth period contains 18 elements and is also a long period.
- The sixth period contains 32 elements and is called a very long period.
- The seventh period contains the remaining elements and is incomplete.
Types of elements in the Modern Periodic Table
1. Representative elements
(s-block and p-block elements) - Groups 1, 2, 13, 14, 15, 16, 17
2. Transition elements
(d-block elements) – Groups 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12
3. Inner transition elements
(f-block elements) – Group 3 Lanthanides and Actinides
4. Inert gases (or noble gases)
The elements of the Zero group, the 18th vertical column, are known as inert gases or noble gases.
Merits of the Long Form of the Periodic Table
It is based on the atomic number, which is a more fundamental property compared to atomic mass. 2. The position of the element in the table is related to its electronic configuration. 3. It shows regular changes in the properties of various elements when moving across a period or down a group.
Defects of the Long Form of the Periodic Table
- The position of Hydrogen is not satisfactory, as its properties relate to Group IA as well as to Group VIIA.
- It could not accommodate the inner transition elements, i.e. Lanthanides and Actinides, in the main body of the periodic table.
- A few elements are not arranged according to their electronic configuration.
Characteristics of the Modern Periodic Table
1. Number of shells and valence electrons
(a) Down a group, i.e. from top to bottom
The number of shells increases successively, i.e. one by one such that the number of shells that an element has equals the number of the period to which that element belongs.
(b) Across a period, i.e. from left to right
On moving from left to right in a given period, the number of shells remains the same. For example, in the third period, the number of shells remains three, i.e. equal to the number of the period.
2. Valency
- Valency denotes the combining capacity of the atom of an element. It is equal to the number of electrons an atom can donate or accept or share.
- On moving down in a given group, the number of electrons in the outermost shell, i.e. valence electrons, remains the same.
- In a given period, the valency of the elements, with respect to Hydrogen, increases arithmetically from 1 to 4 and back to 1.
|
Elements of 2nd period |
Li |
Be |
B |
C |
N |
O |
F |
|
Hydrides of elements |
LiH |
BeH2 |
BH3 |
CH4 |
NH3 |
H2O |
HF |
|
Valency w.r.t. hydrogen |
1 |
2 |
3 |
4 |
3 |
2 |
1 |
3. Properties of elements
The elements in a given group possess similar electronic configuration because the number of electrons in the respective outermost shells is the same. They have similar physical and chemical properties, which change uniformly.
In a period, the number of electrons in the valance shell changes; therefore, the properties of elements in a period differ significantly.
4. Atomic size (atomic radii)
It is the distance between the centre of the nucleus of an atom and its outermost shell.
Trends in Atomic Size
(a) Down a group
In a group, the size of an atom increases as one proceeds from top to bottom. This is due to the successive addition of shells as we move from one period to the next in a group.
(b) Across a period
In a period, the size of an atom decreases from left to right. This is because the nuclear charge increases from left to right in the same period, thereby bringing the outermost shell closer to the nucleus.
5. Metallic character
Elements which have a tendency to lose their valence electrons and form a positive ion are considered as metals.
Trends in metallic character
(a) Down a group
On moving down a group, the increased atomic size is greater as compared to the increased nuclear charge. Therefore, the metallic nature increases as one moves down a group, i.e. an atom can lose electrons easily.
(b) Across a period
On moving across a period, the nuclear charge increases and the atomic size decreases, and hence, elements cannot lose electrons easily.
Therefore, the metallic nature decreases across a period on moving from left to right.